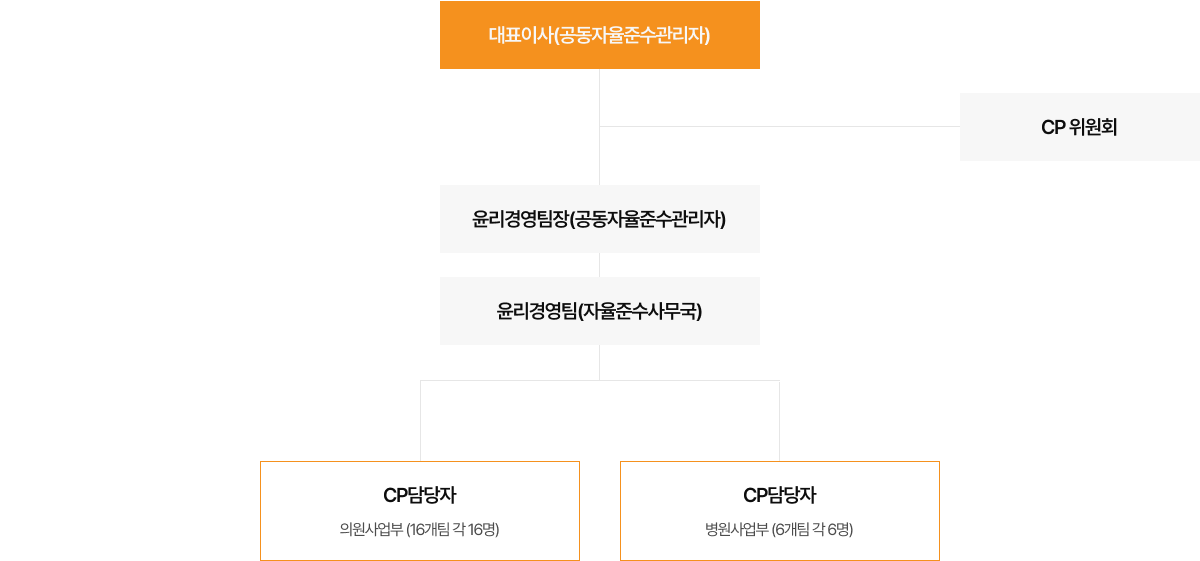
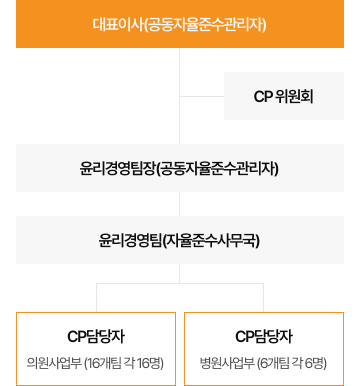
What is the Fair Trade Voluntary Compliance Program?
The Fair Trade Voluntary Compliance Program, also known as the Compliance Program, represents an ‘internal compliance system’. Companies, as economic actors, set up and manage this system themselves. It incorporates education and oversight to ensure businesses promote competitive standards and willingly adhere to fair trade-related laws and regulations (Law and Regulation).
The Compliance Program (CP) furnishes employees with clear guidelines on adhering to competition laws. By instilling a culture of compliance within the company, CP not only facilitates the early detection and voluntary rectification of legal infractions but also provides the groundwork for strategies to prevent recurrent violations.
The Compliance Program (CP) aligns with the Global Standard. Advanced economies like the US, Europe, and Japan have long adopted and operated under CP. It is also incorporated into the criteria used to judge corporate legal transgressions in these regions. Likewise, in our country, CP is considered a pivotal element of corporate economic operations.
Laws Pertaining to Fair Trade
Fair trade-related laws include the Monopoly Regulation and Fair Trade Act, Fair Transactions in Subcontracting Act, Fair Transactions in Agency Act, Fair Transactions in Franchise Business Act, Act on Fair Labeling and Advertising, Framework Act on Consumers, Installment Transactions Act, Act on Door-to-Door Sales, Etc., and the
Act on the Consumer Protection in Electronic Commerce, Etc. All these regulations aim to enhance competition and sustain fair trading standards.